Arsenic or Arsenic in historical films is also called arsenic used to poison. For a long time, people have known about Arsenic and its toxicity.
Learn about Arsenic (Ansen)
According to some studies, arsenic is an extremely toxic substance, 4 times more toxic than mercury. Although in the past, people still used Arsenic commonly in the mining industry, metallurgy, pesticides, herbicides, and even in some weight gain substances for livestock.
Arsenic enters the human body mainly through the respiratory and digestive pathways, especially through the use of groundwater contaminated with Arsenic. Arsenic can cause chronic diseases such as melanoma, epidermal cancer, skin gangrene… It has caused the largest poisoning due to Arsenic accumulation in human history (in Bangladesh). According to research in Japan, through clinical tests, out of 29 people who drank well water contaminated with Arsenic, 23 people (93%) showed signs of melanosis and 22 people (26%) showed signs of melanosis. ) has congenital plantar hyperkeratosis (palmoplantar hyperkeratoris).
In Vietnam, water in some large and densely populated areas such as Hanoi, Viet Tri-Lam Thao, upstream of Ma River… has Arsenic content exceeding the allowed standard, even in some places exceeding 10 times compared to TCVN (allowed Arsenic content in Vietnam’s domestic water is 0.05mg/l). Because in Vietnam, a large number of people still use drinking water, river and stream water for living and eating, so the problem of Arsenic pollution is really urgent. With today’s complicated water use, as well as potential Arsenic contamination that has not yet been detected or investigated, potential poisoning is very likely to occur. In particular, it is even more dangerous when currently, most diseases caused by Arsenic poisoning still have no specific treatment.
To address the extremely urgent needs of treating Arsenic pollution in water sources, we must have a thorough understanding of the chemical cycle of Arsenic in nature to come up with appropriate measures to prevent it. Prevent and limit Arsenic pollution in water sources.
Some properties of Arsenic
An accounts for 1.10-4 % of the total number of atoms in the earth’s crust, they exist mainly in the form of sulfide minerals: Yellow Sulfide Orpiment – As2S 3 and Red Realgar – As4S4;…
In the periodic table of chemical elements, Arsenic is in group Va with a number of characteristics:
Chemical Properties Table of Arsenic Atoms.
| Chemical symbols | As |
| Z | 33 |
| e Configuration | [Ar]3d104s24p3 |
| Rn/death (AO) | 1.48 |
| Rion E3- (AO) | 1.92 |
| Rion E5+ (AO) | 0.47 |
| Eharmonic ion I (kcal/ntg) | 226 |
| EII ion (kcal/ntg) | 466 |
| Eharmonic ion III (kcal/ntg) | 653 |
| Electronegativity | 2.0 |
| Density (g/cm3) | 5,727 |
| TOnc(OC) | 817 |
| TOs (OC) | 614 |
As exists in two forms, metallic and non-metallic:
In its non-metallic form, As is a yellow solid (also known as yellow As) created when condensing vapor, has a cubic network (like white Phosphorus), the network architecture includes As molecules. sub>4 are linked together by Vanderwaals forces. The As4 molecule has a regular tetrahedral structure with As atoms located at the apex. Due to the molecular network, yellow As is less stable at normal temperatures and easily changes to a metallic form (more stable form) under the influence of light.
The metallic form is silver-white, slightly gray in color (called gray As). Gray ash has a polymer structure, has an atomic network similar to black phosphorus, has the ability to conduct heat and electricity but is brittle and can be ground into powder easily.
As exists at oxidation levels -3, +3, +5 with As(III) compounds (Arsenide, Metal Arsenides, Arsenic(III) oxide – As2O3, Arsenic Acid, Arsenic sulfide: As4S6. Arsentrihalide: AsX3) and As(V) compounds (Arsenic oxide : As2O5 ;Arsenic Acid ;Arsenic Sulphide : As2S5 ; Arsenic Pentahalogenide : Only AsF5).
Origin and cycle of Arsenic in nature
- Natural source:
+ Hydrothermal process, magma activity, volcanic activity (most important),
+ Weathering process, erosion process, creating sulfur ore, polymetallic, gold,
+ Alluvial and sedimentation processes lead to the formation of As-containing sediments in the strata
+ The process of deoxidization and dissolution of As-rich minerals in soil into groundwater.
- Artificial sources:
+ As ore processing processes, As extraction from ores…; Metal smelting (especially Cu), Pb, Zn, Ca, Sb, metallurgy
+ Manufacturing and production of plant protection chemicals (pesticides, herbicides, insecticides):
+ In the leather tanning industry, drying agent and wood preservative;
+ In the glass industry;
+ Unscientific exploitation of underground water;
+ The process of burning fossil fuels and solid waste in industry.
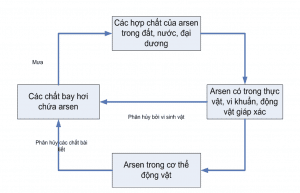
Arsenic cycle is simple
Characteristics and distribution of Arsenic in the natural environment
As predicted, arsenic forms due to the oxidation of arsenopyrrite in clay or peat layers interbedded between them, or because arsenic-rich water from faults cutting through arsenic-rich magmatic rocks in remote mountainous areas seeps into the aquifer. Groundwater in the plains.
Possibility of Arsenic pollution in the delta
Chemical form of Arsenic in the environment
The forms of As present in the environment are a matter of concern because of the differences in toxicity between them. In the environment, As exists mainly in the following forms:
Arsenite As(III), arsenate As(V), arsenious acids (H3 AsO3 , H2AsO3 </ sub>–, HAsO32–) arsenic acids (H3AsO4</sub >, H2AsO4–, HAsO42–), dimethylarsinate (DMA), monomethylarsonate(MMA), arsenobetaine(AB) and arsenocholine (AC).
These types of compounds illustrate the variety of oxidation states of As and the resulting complexity of its chemistry in the environment.
In the aqueous phase with acidic aerated environment, Arsenic predominates at extremely low pH (pH<2), in the pH range 2 – 11 they are replaced by H2AsO 4– and HAsO42–.
Arsenic transmission routes
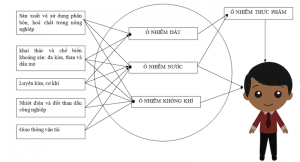
Arsenic transmission pathways
According to studies of people living in areas with As content in well water higher than 0.05 mg/l, up to 20% of the population has dark skin, thickened epidermis and skin cancer. There is currently no effective method to treat As poisoning.
Normally, Arsenic enters the human body in a day and night through the food chain at about 1 mg and is absorbed into the body through the stomach but is also easily excreted. The As content in the human body is about 0.08-0.2 ppm, the total amount of As in a normal person is about 1.4 mg. As is concentrated in the liver, kidneys, red blood cells, homoglobin and is especially concentrated in the brain, bones, skin, lungs, and hair. Currently, people can rely on the As content in the human body to understand the living situation and environment, such as the average As content in the hair of rural population groups is 0.4-1.7 ppm. , industrial city areas 0.4-2.1 ppm, and heavily polluted areas 0.6-4.9 ppm.
As poisoning, also known as Arsenicosis, appears as an environmental disaster for human health around the world. The first symptoms of As poisoning are skin darkening (melanosis) and epidermal thickening (keratosis), which can lead to gangrene or skin cancer. There is currently no effective method to treat As poisoning. Since the 1970s and especially in the past decade, many other negative health effects have been associated with increased As exposure. There is extensive evidence of these effects including peripheral vascular disease (black leg).

– Through the skin: Our skin is about 1% pores and 5-10% water so As can penetrate through the skin into the dermis, dermis and into the blood ; When As penetrates inside, it will destroy cells, prevent biochemical processes of cells, inhibit cell activity and thereby mutate genes, causing cancer.
– Through the respiratory tract: The human lung has an area in contact with air of 40m2, in addition there is a strong ciliary network with an area of more than 140m2; Blood flows through the lungs through these capillaries and facilitates the absorption of pollutants. The result is lung damage: inflammation, irritation, edema, pulmonary fibrosis, lung cancer…
– Through the intestinal membrane : Penetrates into the body through food, followed by absorption. Compounds with low polarity and lipophilicity will dissolve in fat, then diffuse through fat membranes into the blood. Highly polarized metal ions will dissolve in the fluid of the intestinal membrane and affect the cells.
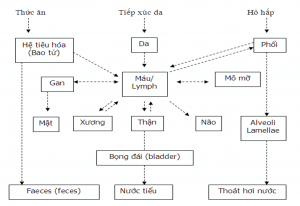
Mechanism of action of Arsenic in the human body
As is a particularly essential element (when in very low concentrations) and an extremely powerful poison (when in high enough concentrations) for the human body and other organisms.
– The longer the exposure and the higher the arsenic concentration, the more cells die.
The toxic mechanism of arsenic is that it attacks the sulfurhydryl groups of enzymes, hindering the enzyme’s activity. Arsenic (III) at high concentrations coagulates proteins because arsenic (III) attacks the bonds containing sulfur groups. When entering the body, especially As(III) immediately attacks enzymes containing the –SH group and hinders their activity.
Arsenate is like phosphate, easy to precipitate with metals and less toxic than arsenite. When entering the body, Arsenate will take the place of phosphate in the chain of reactions to create adenosinetriphosphate (ATP) – a substance that plays an important role in metabolism. cellular metabolism – therefore ATP will not be formed.
In the presence of arsenate, the main biochemical effect that itself produces coagulation of proteins, creates complexes with coenzymes and destroys the phosphatase activity to produce ATP. In summary, the main biochemical effects of arsenic are: protein coagulation; forms complexes with coenzymes and destroys the process of phosphorylation that produces ATP.
In the ecological environment, the divalent form of As compounds (3) is more toxic than the divalent form (5). The reducing environment is a favorable condition for many pentavalent As compounds to convert to trivalent As.
Some symptoms of Arsenic poisoning
– Acute poisoning:
+ Due to absorption of toxic Arsenic through the digestive tract: common symptoms are digestive disorders (abdominal pain, vomiting, burns, dry mouth, severe diarrhea and dehydration…) which can lead to death.
+ Due to breathing air containing dust, smoke or vapor containing As: Common symptoms: Neurological disorders such as headache, dizziness, pain in the limbs. Digestive disorders often occur slowly compared to cases of arsenic ingestion, with symptoms of nausea, vomiting, diarrhea, and abdominal pain. The phenomenon of facial cyanosis. Eye lesions such as eyelid dermatitis, conjunctivitis…
+ When skin comes into contact with Arsenic; Can cause contact dermatitis, folliculitis, ulcers…
– Chronic poisoning :The appearance of dark spots on the body or on the tips of the limbs, tongue mucosa or skin keratinization (usually appears on the hands, feet, parts of the body that are rubbed too much or exposed to a lot of light), can cause necrosis, gradual loss of each toe… eventually leading to cancer, genetic mutation and death.
Recognizing Arsenic in daily life
Properties of Arsenic
Arsenic does not cause an unpleasant odor when present in water, even though its amount can be deadly, so it is difficult to detect by the senses. Therefore, to know the level of arsenic pollution in groundwater, we have the following ways:
– Take samples and bring them to the Institute of Environmental Sciences, Department of Geology and Mineral Resources, medical facilities… for analysis
– Detecting Arsenic contaminated water with glowing bacteria:
A research team from the Swiss Institute of Environmental Science and Technology took advantage of Escherichia coli bacteria’s ability to be sensitive to arsenic to genetically engineer them to glow when arsenic is detected in water. The above success could save the lives of many people using groundwater contaminated with this natural poison. E.coli is also currently being tested in Vietnam, at low cost without releasing toxic chemicals into the environment.
– Arsenic test kit :
According to Dr. Tran Hong Con, Department of Chemical Technology, University of Natural Sciences, Hanoi National University, arsenic in water cannot be detected through the senses. Even water that is clear and feels clean can still contain this toxin. Boiling and filtering bacteria also cannot remove arsenic, manganese and some other heavy metals.
With the Institute of Geology’s arsenic test kit, it only takes 7 minutes to detect whether there is arsenic toxicity in water or not. The kit costs 150,000 VND and can be tested 25 times. With this kit, it is possible to determine Arsenic content in water from 0.005mg/l to 1.5mg/l.
The kit includes a reaction bottle, a bottle of Arsenic indicator paper, a bottle of reducing powder for 25 tests, a bottle of As-1 solution and a pint. All contained in a plastic bag the size of a hand. The user only needs to put the indicator paper in the lid of the reaction vial, pour the water sample, solution and reducing powder into the reaction vial according to the instructions and then close the lid tightly. If the indicator paper turns yellow, it means the water is contaminated with arsenic.
Some Arsenic treatment technologies
Precipitation
Using chemical solutions, create precipitates through chemical reactions with ions dissolved in the solution.
– Iron usually exists in groundwater in the form of dissolved hydrocarbonates. When exposed to oxygen, it will be oxidized to form a precipitate. Long-term deposition will cause the As in the water to combine and settle to the bottom with the iron.
- Advantages
- Arsenic can be treated thoroughly and quickly
- Disadvantages
- Consumes a lot of chemicals
- May block structures behind
- Effects on water quality after treatment
Flocculation
This method includes chemical reactions, the formation of flocs that break the stability of the dirt particles, the adhesion and increase in size of dirt particles in the water to be treated… This method is often used to separate colloidal compounds (size 0.001-100 µm) and dispersed particles suspended in water (>100 µm).
The flocculation method converts As from soluble to insoluble form through a chemical reaction, then separates them from water through sedimentation or filtration.
Commonly used technology is: adding coagulants such as iron or aluminum salts, which can pre-oxidize and adjust pH. In this technology, treating waste residue containing As from the flocculation process is a matter of concern.
Lang
Separation of solid and liquid phases by the effect of gravity.
This method is often used in combination with precipitation and sedimentation.
Filter
Conventional filtration methods are used to separate solids from water by passing water through filter media made of sand, anthracite, activated carbon, filter cloth, and filter paper. These materials allow water to pass through and keep dirt on their surface. Normally this method can separate particulate contaminants such as mud, clay, colloidal particles, small particles from natural organic substances, precipitated compounds of iron and manganese, flocs, bacteria…
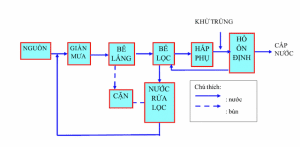
Arsenic filtration process
Adsorption
With water sources with high minerality, the adsorption method is effective due to the specific interactions of the system. The adsorbent materials used are some metal oxides such as aluminum, iron, manganese, or a mixture of the above oxides. Arsenic (arsenate form) adsorbs on the above oxide materials according to many authors by the mechanism of creating surface complexes on solids. Accordingly, before creating chemical bonds they are adsorbed and this is usually the slowest stage of the process.
- Advantages
- Less affected if sulfate and soluble residue content (total ion concentration)
- -Al2O3 has selective adsorption properties for arsenic compounds
- Disadvantages
- Difficulty in regeneration -Al2O3
Oxidize
A relatively simple method, allowing oxygen to act and take over electrons in the atoms of the reactant. Aerate by blowing air into the water, creating FeAssO4. Photochemical oxidation reaction is an oxidation reaction that occurs in a chemical system thanks to radiation energy.
Solar Oxidation and Removal of As (SORAS):
This is a simple As treatment process in rural water supply from underground water sources, using the photochemical oxidation reaction of As+3 to As+5 thanks to light. sunlight, then separate As+5 from water by absorption with Fe+3 particles. The photochemical oxidation reaction is enhanced by adding a few drops of lemon or concentrated lime water, which helps in the formation of Fe+3 flocs. SORAS is effective when the iron content in groundwater is at least 3mg/L, UV-A radiation intensity50Wh/m2.
Solar distillation:
This method uses solar energy to evaporate water, then condenses the water. The process of evaporation and condensation of water will separate all substances, including As, from water. In countries in the tropics, with available solar energy sources, this method may be suitable, especially in heavily polluted areas. Research can be applied to rural areas if the problem of economic efficiency can be solved.
Membrane filtration:
Use semi-permeable membranes, which only allow water and some solutes to pass through to purify the water. Membrane filtration technology allows any type of dissolved solids to be removed from water, including As.
However, this method is often very expensive and therefore is often used in necessary and mandatory cases where it is difficult to apply other methods such as desalination, removal of some ions such as As, etc. There are many filter membranes that can be used. used as microfiltration, reverse osmosis, electrodialysis, ultrafiltration and nanofiltration.
Plant filtration:
This is a new technology. According to American scientists, it is possible to clean arsenic-contaminated water by growing a fern called Pteris vittata. In less than a day, plants will absorb As from the water, causing the level of this toxic metal to drop below the safe threshold set by the agency27.
Set by the US Environmental Protection Agency (EPA). This is a new, inexpensive method and the above fern grows in hot and humid climates like the climate in many parts of Asia. Unlike most other As removal methods, phytofiltration of As does not create sludge rich in As that is difficult to dispose of. Pressing fern plastic can extract 70% of As for industrial purposes.
Various research results show that both the Typha latifolia strain in Bangladesh and the Typha orientalis strain in Vietnam have the ability to strongly and quickly absorb arsenic, thereby reducing the toxic content in water below safe levels. 0.01mg/liter. In the South, candle grass called tub tub is often found growing wild with a height that rarely exceeds 2 meters and looks like a reed. It is characterized by a set of candle-shaped flowers that the oriental medicine industry exploits under the name of incense stick. Roots and underground stems grow best in flooded areas.
Dr. J.D. Jackson introduced many years of experimental work in Civil
Engineering in April 2007, according to which candle grass absorbs 89%, the arsenic content in well water is only about 38 micrograms, suitable for drinking.

Enzyme method:
The general principle is to use enzymes to catalyze the reaction of converting very toxic Arsenic (III) into less toxic Arsenite (V) or converting inorganic As to organic form. For example, the enzyme arsenate reductase from Alcaligenes faecalis strain catalyzes the reaction that converts arsenite (III) to arsenate (V).
Criteria for choosing suitable technology:
- The quality of water after treatment must meet usage requirements.
- Simple technology.
- Low cost.
- Uses no or minimal power requirements.
- Able to apply to different types of water sources, different water supply capacities, and service scales.
- Use local raw materials and labor.
- Accepted by the community
* The above methods require high costs, complex technology, and are difficult to use in households. At the household scale, measures can be applied to prevent:
The rain rig is made of plastic pipe, 27mm diameter, drilled with 150-200 holes, each hole has a diameter of 1.5-2mm depending on the pump capacity being used. At the bottom of the filter tank is a layer of supporting gravel about 1 gang thick, on top of the supporting gravel layer is a layer of sand about 2.5-3 gang thick. Do not use bedding or charcoal that can easily cause side effects, increasing nitrite levels in the water.
Water from the pump hose flows into the rain gutter. Rain gutters need to have many small holes so that air can easily dissolve into water, promoting the oxidation effect of oxygen available in the air. After passing through the rain gutter, water flows through a filter tank with 3 compartments: the first compartment is used to filter sediment, raw water flows from the bottom up; There is a drain line at the bottom, the second compartment is used to filter water, water flows from the top, the third compartment is used to store clean water. The optimal size of the filter tank depends on the capacity and flow rate of each well. The Center for Clean Water and Environmental Sanitation in Thai Binh province has used this type for a long time.
State management solutions with Arsenic
Local authorities regularly make statistics and review the situation of households using local groundwater, periodically coordinate with functional units to take samples for analysis, and promptly detect changes in arsenic concentrations. in groundwater to take countermeasures.
The state strengthens management of areas where arsenic is generated: in industry (especially in the glass, ceramic, leather, dye and paint industries), use of fertilizers and chemicals. Plant protection.
Warn about the harmful effects of using groundwater containing arsenic on human health, ways of arsenic penetration, and symptoms of arsenic poisoning to keep people alert through mass media and propaganda sessions. transmit.
Strengthen the development of clean water supply for the people, ensuring the concentration of arsenic in drinking water quality in accordance with regulations of the Ministry of Health.
Disseminating simple, low-cost arsenic removal techniques that can be applied in areas where people currently do not have access to clean water and are using underground water sources containing arsenic

 Tiếng Việt
Tiếng Việt


Related articles
Nam Viet Environment Company cleans up beach trash to spread the message of environmental protection
No one alone can litter a pile of trash. Anyone who sees trash criticizes it,...
Common Aerotank tank problems and how to fix them
Aerotank incidents during the operation of the WWTP often arise equipment problems such as pumps,...
Unitank tank in wastewater treatment
Introducing the Unitank tank The simplest structure of a Unitank tank is a rectangular tank...